
Large cell carcinomas, on the other hand, grow and spread quickly, which can make them difficult to treat.Īdvanced cancers are harder to treat, as they are more likely to have spread before a doctor can diagnose a person. However, there are some differences.Īdenocarcinomas tend to grow in the outer edges of a person’s lungs, and doctors are more likely to find them before they spread to other organs. The ACS adds that it groups them together as they share similar treatments and outlooks.
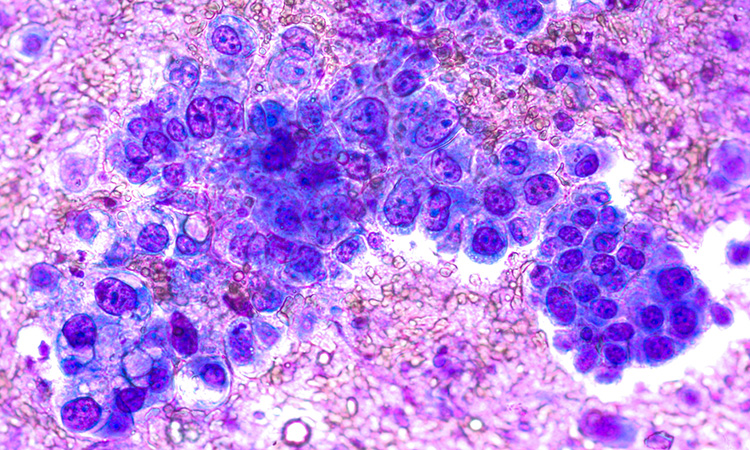
The ACS explains that there are three main types: doi:10.Share on Pinterest Inside Creative House/Getty ImagesĪccording to the Centers for Disease Control and Prevention (CDC), NSCLC is the more common type of lung cancer. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Jacobsen CA, Chavez JC, Sehgal AR, et al. Real-world early outcomes of axicabtagene ciloleucel for relapsed or refractory (R/R) follicular lymphoma (FL). However, those who would have been eligible for ZUMA-7 showed lower rates of grade 3 or higher ICANS and quicker ICANS resolution.ġ.
#REAL ALTERNATIVE MEDICINE FOR SMALL CELL LUNG CANCER TRIAL#
Notably, PFS and OS at 6 months post-treatment were similar regardless of patient eligibility by ZUMA-5 trial criteria. Among 150 patients who were alive at day 30, prolonged cytopenia occurred in 11%. The median cumulative incidence estimates of CRS and ICAN resolution were 5 days and 4 days, respectively. Jacobson, MD, MMSc, medical director of the Immune Effector Cell Therapy Program at Dana-Farber Cancer Institute and assistant professor of medicine at Harvard Medical School.ĬRS considered grade 3 or higher by ASTCT consensus occurred in 2% of patients (95% CI, 0% to 6%), and immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 13% of patients (95% CI, 8% to 19%). “Despite a broader patient population and relatively limited follow-up in the real world, early results demonstrate favorable effectiveness and safety outcomes with axi-cel in patients with relapsed or refractory follicular lymphoma that are consistent with those observed in the pivotal ZUMA-5 trial, supporting postauthorization use of this CAR T-cell therapy in this setting,” said lead study author and presenter Caron A. The estimated progression-free survival (PFS) and overall survival (OS) were 88% (95% CI, 81% to 92%) and 96% (95% CI, 91% to 98%). Patients who received previous nontransplant cellular therapy, including prior CAR T-cell therapy, were excluded.Īmong 151 patients with follow-up data at a median of 6.2 months, the ORR was 93% (95% CI, 88% to 97%) and the CR rate was 84% (95% CI, 77% to 89%). Patients had a median of 4 lines of therapy prior to receiving axi-cel, 14% had undergone prior autologous stem cell transplantation, and 9% received bridging therapy. The median patient age was 62 years, and 60% of patients in the study were male.
Of 230 patients in the real-world study population, 40% would have been excluded from the ZUMA-7 trial, mainly due to comorbidities. In the new analysis, the patient population is significantly broader than that of the ZUMA-5 trial.

2 Regarding safety, cytokine release syndrome (CRS) grade 3 or higher occurred in 6% of patients, and neurologic events occurred in 15% of patients. In the pivotal ZUMA-5 trial of axi-cel in R/R FL following at least 2 lines of therapy, the primary analysis showed a 94% objective response rate (ORR) and a 79% complete response (CR) rate in patients treated with axi-cel. 1 The findings were presented during a poster discussion session at the 2023 American Society of Clinical Oncology Annual Meeting. In the largest report of real-world axicabtagene ciloleucel (axi-cel) outcomes among patients with relapsed or refractory follicular lymphoma (R/R FL), including patients who would have been ineligible for the pivotal ZUMA-5 trial, the chimeric antigen receptor (CAR) T-cell therapy showed safety and efficacy consistent with trial outcomes.


 0 kommentar(er)
0 kommentar(er)
